
In solution, exposed to air, the hydroxide ion reacts rapidly with atmospheric carbon dioxide, acting as an acid, to form, initially, the bicarbonate ion. A consequence of this is that concentrated solutions of sodium hydroxide have high viscosity due to the formation of an extended network of hydrogen bonds as in hydrogen fluoride solutions. In aqueous solution the hydroxide ion forms strong hydrogen bonds with water molecules. This compound is centrosymmetric and has a very short hydrogen bond (114.5 pm) that is similar to the length in the bifluoride ion HF −Ģ (114 pm). Indeed, the bihydroxide ion HĢ has been characterized in the solid state.
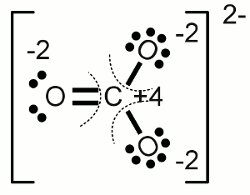
In aqueous solution both hydrogen and hydroxide ions are strongly solvated, with hydrogen bonds between oxygen and hydrogen atoms. It can also act as a Lewis base by donating a pair of electrons to a Lewis acid. In aqueous solution the hydroxide ion is a base in the Brønsted–Lowry sense as it can accept a proton from a Brønsted–Lowry acid to form a water molecule. Schematic representation of the bihydroxide ion
pOH can be kept at a nearly constant value with various buffer solutions. For example, ammonia solutions have a pH greater than 7 due to the reaction NH 3 + H + ⇌ NH +Ĥ, which decreases the hydrogen cation concentration, which increases the hydroxide ion concentration. Addition of a base to water will reduce the hydrogen cation concentration and therefore increase the hydroxide ion concentration (increase pH, decrease pOH) even if the base does not itself contain hydroxide. The concentration of hydroxide ions can be expressed in terms of pOH, which is close to (14 − pH), so the pOH of pure water is also close to 7. The pH of a solution is equal to the decimal cologarithm of the hydrogen cation concentration the pH of pure water is close to 7 at ambient temperatures. Has a value close to 10 −14 at 25 ☌, so the concentration of hydroxide ions in pure water is close to 10 −7 mol∙dm −3, in order to satisfy the equal charge constraint. The equilibrium constant for this reaction, defined as The hydroxide ion is natural produced from water by the self-ionization reaction: H 3O + + OH − ⇌ 2H 2O Many inorganic substances which bear the word hydroxide in their names are not ionic compounds of the hydroxide ion, but covalent compounds which contain hydroxy groups. The corresponding covalently bound group –OH of atoms is the hydroxy group.īoth the hydroxide ion and hydroxy group are nucleophiles and can act as catalysts in organic chemistry. The corresponding electrically neutral compound HO Sodium hydroxide is a multi-million-ton per annum commodity chemical. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. It functions as a base, a ligand, a nucleophile, and a catalyst. It is an important but usually minor constituent of water. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge.

Hydroxide is a diatomic anion with chemical formula OH −.


 0 kommentar(er)
0 kommentar(er)
